12. The Sources and Causes of Soil P Losses and the Role of RPR in Reducing Them


RPR Revisited 5:
The Sources and Causes of Soil P Losses and the Role of RPR in Reducing Them
1Bert F. Quin and 2Gordon Rajendram
1Quin Environmentals (NZ) Ltd
2Eurofins NZ Ltd
Presented to the
New Zealand Soil Science Society Conference
Napier, New Zealand, 3-6 Dec 2018
P Leaching
-
The key drivers of P leaching from soils are the degree of saturation of the soil’s P sorption capacity, and the volume of drainage.
-
The higher the percentage of saturation of the P sorption capacity (Pscs%), the greater the concentration of P maintained in the soil solution, and therefore the greater the losses of P in any drainage (Sharpley 1995). This applies to both Pi and Po.
-
Saturation occurs gradually through either the diffusion of P into soil mnerals via imperfections in their surfaces (Fig.1a), or its occlusion by being coated by Fe and Al oxides while physically bound to soil mineral surfaces (Fig.1b).
Figure 1: The mechanisms for the gradual saturation of soil solution P sorption capacity
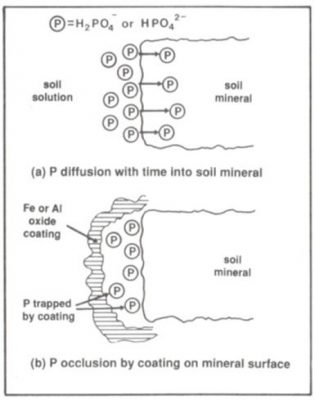
RG McLaren, KC Cameron: Soil Science, 1990, pg 211-212
The Pscs%
-
The percentage saturation of the phosphate sorption capacity (Pscs%) is very important environmentally, but unlike the Netherlands for example, New Zealand soils and farms are not tested for this.
-
Instead, only a relative assessment of the P sorption capacity of the soil is made (the phosphate retention or PR test, expressed on a 0-100 scale) for use in assessing maintenance P.
PR or ASC – what’s in a name?
-
The PR test currently is used only to help assess required maintenance P inputs and soil Olsen P levels to maintain a given level of farm production. It was renamed the Anion Storage Capacity (ASC) about 15 years ago.
-
The term ASC is a misnomer and should never have been adopted. A ‘store’ or ‘storage’ is by definition somewhere you deliberately put something until such time that you want to get it out to use it.
-
When we apply soluble P fertiliser however, we have little or no control over the rate it which it will be adsorbed (into ‘storage’), or how quickly the soil P concentration will reach equilibrium again.
-
Just as importantly, the rate of desorption into plant available form is rarely capable of maintaining optimum production for more than 2 or 3 years after fertiliser is withheld.
-
So this sorbed P is not a ‘store’ at all; it is better described as a bank ‘savings’ account which unfortunately pays no interest and can only be withdrawn at rates that are far too low to maintain a reasonable state of living.
The goal; a dual-purpose soil agronomic and environmental management test
-
Where soluble P is used, the concentrations of P in the soil water are generally high enough to give rise to eutrophic levels of P in any drainage. Only 0.015 mg/L dissolved reactive P (DRP) is required for drainage water to be eutrophic. This represents only 0.1 kg/ha with an annual drainage of 400mm; not economically significant, but enormously important environmentally.
-
Unfortunately, the Olsen P test, while reasonably useful agronomically, is by itself far to blunt an instrument to be used in environmental management.
-
Measurements of CaCl2-P in drainage water have shown ‘change-points’ near the top of the pasture production response curve, above which losses of P in drainage water accelerate (Fig. 2, McDowell 2012). But the change-point varies markedly with different soils, so this approach has little practical benefit.
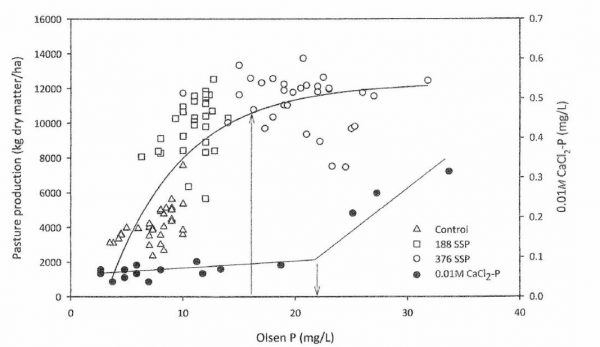

-
However, McDowell and Condron (2004) had already produced a rather snazzy predictor of DRP in drainage from the Olsen P and PR over a range of soils, viz:
DRP = 0.069 (Olsen P/PR) + 0.007
-
The Olsen P/PR ratio is essentially an expression of the degree of soil P sorption capacity. Very importantly, it bridges the gap between purely agronomic soil testing and environmental management.
-
It is disappointing that more has not been done to promote the use of the ratio in environmental management.
-
Setting a limit of say 0.35 for the Olsen P/PR ratio for pastoral soil development throughout New Zealand would bring major improvements to water quality, without significantly reducing farm production.
Relevance to RPR
RPR has been proven to maintain any given level of pasture production with considerably lower Olsen P levels on most soils, by many researchers (Fig. 3a).
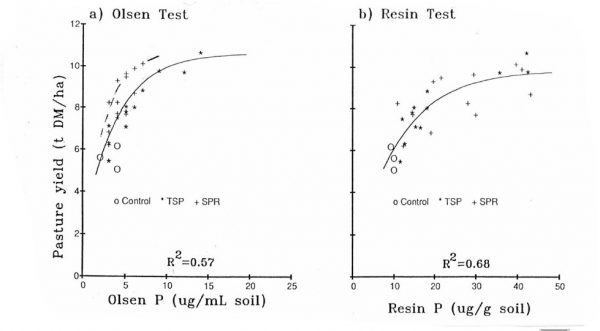
-
This is a consequence of the fact that far more of the P uptake from RPR is coming more directly from gradually dissolving particles of RPR. The Resin P test (Fig. 3b) provides a far better assessment of this drip-feed.
-
When soluble P is applied however, soil water P concentrations around dissolving granules can reach hundreds of mg P/L, overloading plant P uptake requirements and soil sorption rates for a period of weeks or months. During this pre-equilibrium period, large P leaching losses are possible from many soils, with phosphate retentions as high as 50%.
-
This overloading and resulting susceptibility to loss does not occur with RPR. It is important to note however the fact that not all phosphate rocks can be used as direct application P fertilisers capable of maintaining high levels of pasture production.
-
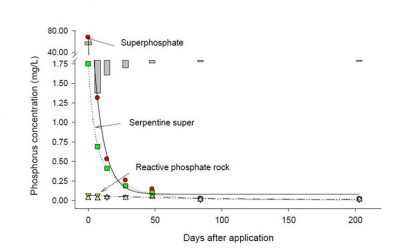
Only reactive phosphate rocks or RPRs, as a function of having at least 20% substitution of phosphate by carbonate in the crystal lattice (which gives them a much higher solubility product in mildly acid soils), can maintain sufficient concentrations of P in the soil solution for vigorous pasture production (Figure 4).
Figure 4
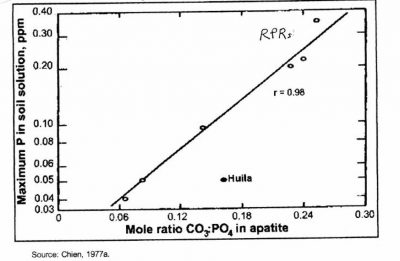
P run-off
-
Dissolved Reactive P (DRP). The concentration of DRP in run-off is driven very largely by specific fertiliser applications and by the form of P applied.
-
Many studies around the world, including several in New Zealand (eg McDowell and Catto 2005), have demonstrated the very high levels of DRP that can occur in the first 2 or 3 run-off events after application of soluble P (Fig.5). This reflects the very high levels of soluble fertiliser P in the near-surface soil water.
Figure 5. P concentrations in surface runoff: Water soluble P versus non water soluble P

-
10 kg P/ha or more can be lost in single run-off events (eg Nash 1998). This can occur even when a run-off occurs months after application, if prior soil moisture levels have been too low for complete dissolution of fertiliser granules and the movement of P into the soil.
-
These losses of DRP simply do not occur with RPR.
-
This raft of evidence should have lead to RPR being strongly recommended for use in all sensitive catchments by soil fertility research scientists. Unfortunately, the potential role of RPR to mitigate P run-off has been deliberately reduced in recent publications from NZ.
-
RPR’s overall ‘ranking’ as a P run-off mitigation option was reduced to a poorly justified ‘0-20%’ by McDowell (2012), lower than many of other mitigations listed, including flood irrigation management, sorbents in and near streams, dams and water recycling, applying alum and red mud to pasture etc, pure clover swards in sensitive areas, not applying fertiliser P to ‘hot-spots’ etc. And who pays for installing and maintaining all this?
-
The fact that the unnecessary application of soluble P in the first place is the root cause of virtually all P loss is no longer mentioned in published papers by scientists funded under the Mitigator project; nor is it in the ‘public’ version of Ballance’s Mitigator® webpage.
-
Instead of promoting the one-step practical solution of switching from soluble P to RPR, which alone of all mitigations saves the farmer money as well, a raft of expensive and site-unproven ‘remedies’ that are costly for the farmer to install and maintain are being presented.
-
One excuse given for not promoting the benefits of using RPR as a P run-off loss mitigation is results from studies showing that while DRP losses are greatly reduced with RPR, particulate P losses are not. Despite DRP making up 30-70% of total P losses in most cases (Hart et al 2004), the attitude now appears to be ‘why bother using RPR if particulate P losses are not reduced also?’
-
This logic, as well as being deeply flawed in itself, ignores the fact that all particulate P loss comparisons between soluble P and RPR have been done on areas with a background of soluble P use.
-
Trials sites that have not had a history of RPR applications cannot be used to assess the effect of particulate P loss with RPR for a number of reasons:
-
RPR particles are much denser (Bulk Density 1.65) than soluble P (BD 1.0-1.1), and are therefore far less prone to being carried off in run-off.
-
This density difference also means that RPR particles ‘sink’ into the soil must faster, further reducing their susceptibility to run-off.
-
As seen in Fig. 4, RPR is not capable of producing P concentrations in soil water much greater than 0.2 mg/L.
-
The use of soluble P however produces very high concentrations of weakly adsorbed ‘Olsen’ P near the soil surface, often much higher than in the standard 0-75mm sampling depth.
-
Much (>25%) of this loosely-bound P in near-surface soil is easily desorbed when the soil particles enter a body of water, for equilibrium reasons demonstrated decades ago by Australian researchers (eg Barrow 1983).
Conclusions
-
There is overwhelming evidence that the use of RPR instead of soluble P would greatly reduce both P leaching and the loss of soluble P in run-off events.
-
The advantage of RPR is likely to apply to particulate P losses as well, on farms where RPR has been used for a period of years.
-
There needs to be greater focus on introducing P soil tests that are useful for environmental management rather than just productivity advice. The introduction of limits on the Olsen P to PR ratio, for example a ratio of 0.35, would have very considerable environmental benefits.
-
Ways must be found to create a soil fertility research environment in NZ that is far more open to discussion and scrutiny than is currently the case. Approval of research topics, the publishing of trial data, and the availability of environmentally-protective products are under the control of profit-focused management staff of the two superphosphate-manufacturing cooperatives.
-
Instead of hiding behind Mitigator® and the huge expenditure of farmers money on it, scientists involved should at the very least be openly stating why they are not supporting the use of RPR as a practical and effective P-loss mitigation, given despite the huge amount of evidence in favour of this benefit.
